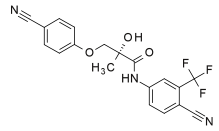
Ostarine
((2S)-3-(4-cyanophenoxy)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydroxy-2-methylpropanamide)
Imagine a compound that elicits steroid like muscle building effects with little or no androgenic side effects, a compound that packs on lean body mass while lowering body fat in individuals who don't even weight train, a compound that is safe to use for months and may even be used by females because it does not cause females to develop male sex characteristics, a compound that makes you feel good, improves sleep, increases libido and changes body composition positively. Well, we don't need to imagine this compound because Osta rx possesses these traits and is a present day reality.
SARM is an abbreviation for Selective Androgen Receptor Modulator. SARMs cause selective anabolic activity in select androgen receptors and not others, hence the term. This nonsteroidal compound has tissue-selective anabolic effects in muscle and bone, while sparing other androgenic tissue related to hair growth in women and prostate effects in men. In other words, SARMs have the potential to offer anabolic effects similar to steroids with little to no common steroidal negative side effects.
SARMs have been studied and developed for over 10 years now by research pharmaceutical company GTx. James Dalton and his colleagues have done numerous studies with hundreds of human participants to determine the viability of SARMs for treating muscle wasting in cancer patients. Typically steroids are prescribed off label to counter muscle wasting diseases but they present several unwanted side effects due to elevations in DHT and Estradiol. Additionally steroids can cause females to develop male characteristics.Therefore a natural need has arisen for an anabolic agent that targets muscle and bone but does not cause other unwanted androgenic activity. SARMs offer an improved benefit to risk ratio for those suffering from muscle wasting.
In August of 2011 a 2006 12-week double-blind, placebo-controlled phase II clinical trial was released to the public absolutely proving that GTx-024 (Ostarine) demonstrated the ability of a nonsteroidal, orally bioavailable SARM to increase lean muscle mass and improve physical function.The conclusion of the study states that;
"There are currently no approved therapies available for the prevention or treatment of muscle wasting. GTx-024 is a novel SARM that was well tolerated in elderly men and postmenopausal women and resulted in significant increases in total lean body mass and improvements in physical function. Importantly, this study provides evidence that GTx-024 provides beneficial anabolic effects on total lean body mass and physical function without the adverse consequences often seen with testosterone and other anabolic steroids. These data support the development of GTx-024 for treatment and prevention of muscle wasting in patients with chronic diseases."
Furthermore the abstract states that;
"GTx-024 showed a dose-dependent improvement in total lean body mass and physical function and was well tolerated. GTx-024 may be useful in the prevention and/or treatment of muscle wasting associated with cancer and other chronic diseases."
In other words, the more Ostarine that was administered the greater the increases in lean body mass.
Ostarine and lean body mass
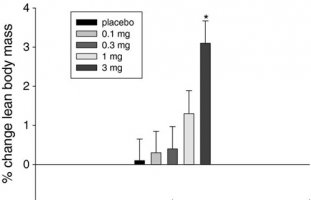
The following chart demonstrates a statistically significant LBM gain of about 3lbs in non-training elderly subjects that were administered a mere 3mg of Ostarine daily for 12 weeks. These subjects also lost almost a pound of body fat at the same time. No differences in total body weight were observed, indicating that the shift to more lean body composition in the 3-mg-dose group was achieved, at least partially, at the expense of body fat.

Fig. 1
Percentage change from baseline to day 86/EOS in total lean body mass: evaluable population. EOS end of study, *P<0.001 3 mg vs. placebo (T test)
Ostarine and power output
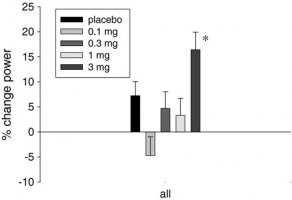
The next chart shows that stair climbing power increased dose dependently as well as the decrease in the time needed to climb the stairs.

Fig. 2
Percentage change from baseline to day 86/EOS in stair climb power: evaluable population. EOS end of study, *P=0.0133 mg vs. placebo (T test)
It is important to note that these doses are quite low (3mg or less) and no strength training was performed by the test subjects. Therefore, a subject who administers a higher dose of Ostarine and performs weight resistance training is likely to increase lean body mass by much more while losing body fat.
Ostarine effects on hormones
In men, no statistically significant differences from placebo in change from baseline values for free testosterone, DHT, estradiol, follicle-stimulating hormone (FSH), or LH were observed at any GTx-024 dose. However, sex hormone-binding globulin (SHBG) was significantly reduced with GTx-024 3 mg versus placebo. The decrease in SHBG was accompanied by a reduction of serum total testosterone in subjects treated with 1 mg or 3 mg of GTx-024 compared to placebo. This data demonstrates that Ostarine is total testosterone suppressive but free testosterone, LH and FSH were not statistically suppressed.
Adverse effects
Ostarine did not cause any increase in hair growth or any increase in skin oil. Often times steroids may cause unwanted hair growth and acne but this is not the case with Ostarine. Several participants in the study had temporary elevations in the liver enzyme ALT however those elevations resolved. HDL did lower slightly but the overall cardiovascular risk/benefit ratio for Ostarine is low. Due to these minor temporary negative effects on lipids and ALT liver enzymes, using a cycle supporting supplement and staying properly hydrated are recommended.
Bone Mineral Density
In this 12 week study, Ostarine showed no difference in bone mineral density compared to placebo. Changes in BMD were not necessarily expected as the treatment period was likely too short to detect a benefit. In preclinical studies, Ostarine demonstrated both anabolic and antiresorptive activity in bone. Future research is warranted as the potential dual beneficial effects of Ostarine and other SARMs on muscle and bone may provide a unique advantage to currently available agents for osteoporosis that solely modify bone strength.
Support ingredients in Osta rx
Osta rx contains an additional complex of compounds that increase libido in both men and women, mood, sense of well being and energy while improving sleep. Although Ostarine has a half life of 24 hours it is recommended that the doses are split during the day due to these other supporting ingredients.
Dosing for Osta rx
Osta rx may be used in male or female users at 3 capsules (20mg Ostarine) per day for up to 8 weeks in duration. Lower doses would allow for longer durations of use.
Osta rx is an exciting product both on paper and in the real world. Here is a summary of recent user feedback.
* Great pumps
* Leaning out
* Added lean body mass
* Vascularity
* Increased libido
* Improved training recovery
* Strength gains
* No reported side effects
* Little to no suppression
* Improved mood and energy
Referrences
The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial.
GTx, Inc. enobosarm (Ostarine or GTx-024) : Preventing and Treatment Muscle Wasting in Lung Cancer Patients
((2S)-3-(4-cyanophenoxy)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydroxy-2-methylpropanamide)

Imagine a compound that elicits steroid like muscle building effects with little or no androgenic side effects, a compound that packs on lean body mass while lowering body fat in individuals who don't even weight train, a compound that is safe to use for months and may even be used by females because it does not cause females to develop male sex characteristics, a compound that makes you feel good, improves sleep, increases libido and changes body composition positively. Well, we don't need to imagine this compound because Osta rx possesses these traits and is a present day reality.
SARM is an abbreviation for Selective Androgen Receptor Modulator. SARMs cause selective anabolic activity in select androgen receptors and not others, hence the term. This nonsteroidal compound has tissue-selective anabolic effects in muscle and bone, while sparing other androgenic tissue related to hair growth in women and prostate effects in men. In other words, SARMs have the potential to offer anabolic effects similar to steroids with little to no common steroidal negative side effects.
SARMs have been studied and developed for over 10 years now by research pharmaceutical company GTx. James Dalton and his colleagues have done numerous studies with hundreds of human participants to determine the viability of SARMs for treating muscle wasting in cancer patients. Typically steroids are prescribed off label to counter muscle wasting diseases but they present several unwanted side effects due to elevations in DHT and Estradiol. Additionally steroids can cause females to develop male characteristics.Therefore a natural need has arisen for an anabolic agent that targets muscle and bone but does not cause other unwanted androgenic activity. SARMs offer an improved benefit to risk ratio for those suffering from muscle wasting.
In August of 2011 a 2006 12-week double-blind, placebo-controlled phase II clinical trial was released to the public absolutely proving that GTx-024 (Ostarine) demonstrated the ability of a nonsteroidal, orally bioavailable SARM to increase lean muscle mass and improve physical function.The conclusion of the study states that;
"There are currently no approved therapies available for the prevention or treatment of muscle wasting. GTx-024 is a novel SARM that was well tolerated in elderly men and postmenopausal women and resulted in significant increases in total lean body mass and improvements in physical function. Importantly, this study provides evidence that GTx-024 provides beneficial anabolic effects on total lean body mass and physical function without the adverse consequences often seen with testosterone and other anabolic steroids. These data support the development of GTx-024 for treatment and prevention of muscle wasting in patients with chronic diseases."
Furthermore the abstract states that;
"GTx-024 showed a dose-dependent improvement in total lean body mass and physical function and was well tolerated. GTx-024 may be useful in the prevention and/or treatment of muscle wasting associated with cancer and other chronic diseases."
In other words, the more Ostarine that was administered the greater the increases in lean body mass.
Ostarine and lean body mass
The following chart demonstrates a statistically significant LBM gain of about 3lbs in non-training elderly subjects that were administered a mere 3mg of Ostarine daily for 12 weeks. These subjects also lost almost a pound of body fat at the same time. No differences in total body weight were observed, indicating that the shift to more lean body composition in the 3-mg-dose group was achieved, at least partially, at the expense of body fat.
Fig. 1
Percentage change from baseline to day 86/EOS in total lean body mass: evaluable population. EOS end of study, *P<0.001 3 mg vs. placebo (T test)
Ostarine and power output
The next chart shows that stair climbing power increased dose dependently as well as the decrease in the time needed to climb the stairs.
Fig. 2
Percentage change from baseline to day 86/EOS in stair climb power: evaluable population. EOS end of study, *P=0.0133 mg vs. placebo (T test)
It is important to note that these doses are quite low (3mg or less) and no strength training was performed by the test subjects. Therefore, a subject who administers a higher dose of Ostarine and performs weight resistance training is likely to increase lean body mass by much more while losing body fat.
Ostarine effects on hormones
In men, no statistically significant differences from placebo in change from baseline values for free testosterone, DHT, estradiol, follicle-stimulating hormone (FSH), or LH were observed at any GTx-024 dose. However, sex hormone-binding globulin (SHBG) was significantly reduced with GTx-024 3 mg versus placebo. The decrease in SHBG was accompanied by a reduction of serum total testosterone in subjects treated with 1 mg or 3 mg of GTx-024 compared to placebo. This data demonstrates that Ostarine is total testosterone suppressive but free testosterone, LH and FSH were not statistically suppressed.
Adverse effects
Ostarine did not cause any increase in hair growth or any increase in skin oil. Often times steroids may cause unwanted hair growth and acne but this is not the case with Ostarine. Several participants in the study had temporary elevations in the liver enzyme ALT however those elevations resolved. HDL did lower slightly but the overall cardiovascular risk/benefit ratio for Ostarine is low. Due to these minor temporary negative effects on lipids and ALT liver enzymes, using a cycle supporting supplement and staying properly hydrated are recommended.
Bone Mineral Density
In this 12 week study, Ostarine showed no difference in bone mineral density compared to placebo. Changes in BMD were not necessarily expected as the treatment period was likely too short to detect a benefit. In preclinical studies, Ostarine demonstrated both anabolic and antiresorptive activity in bone. Future research is warranted as the potential dual beneficial effects of Ostarine and other SARMs on muscle and bone may provide a unique advantage to currently available agents for osteoporosis that solely modify bone strength.
Support ingredients in Osta rx
Osta rx contains an additional complex of compounds that increase libido in both men and women, mood, sense of well being and energy while improving sleep. Although Ostarine has a half life of 24 hours it is recommended that the doses are split during the day due to these other supporting ingredients.
Dosing for Osta rx
Osta rx may be used in male or female users at 3 capsules (20mg Ostarine) per day for up to 8 weeks in duration. Lower doses would allow for longer durations of use.
Osta rx is an exciting product both on paper and in the real world. Here is a summary of recent user feedback.
* Great pumps
* Leaning out
* Added lean body mass
* Vascularity
* Increased libido
* Improved training recovery
* Strength gains
* No reported side effects
* Little to no suppression
* Improved mood and energy
Referrences
The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial.
GTx, Inc. enobosarm (Ostarine or GTx-024) : Preventing and Treatment Muscle Wasting in Lung Cancer Patients