OSTA Rx -- SARM
Selective Androgen Receptor Modulator (SARM)

-Non-hormonal Anabolic Compound
-Increases Lean Muscle Mass
-Promotes Fat Loss
-Promotes Recovery
-Increases Libido
-Safe for Males & Females
-Can be used for PCT and Bridging
IronMagLabs Bodybuilding Supplements & Prohormones: Osta Rx
(MK-2866) ~ ((2S)-3-(4-cyanophenoxy)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydroxy-2-methylpropanamide)
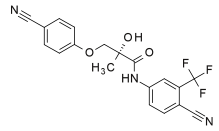
Osta Rx??? is a Selective Androgen Receptor Modulator. A SARM is exactly what it sounds like: a compound (not an anabolic steroid) which has the ability to stimulate the androgen receptor (much the same way as anabolic steroids). Osta Rx??? is an orally active (and highly bioavailable) selective agonist for androgen receptors which was shown to have anabolic effects in muscle and bone tissue. It has been shown to have no measurable effect on lutenizing hormone (LH) or follicle-stimulating hormone (FSH), but it has been shown to have some effect on prostate weight, with an androgenic potency around 1/3rd of its anabolic potency. Still, this is a good trade-off, because it???s anabolic effect has been measured to be roughly the same as testosterone. It has also been shown to produce dose-dependent increases in bone mineral density and mechanical strength in addition to being able decrease body fat and
increase lean body mass.

Selective androgen receptor modulators (SARMs) bind to the androgen receptor and demonstrate osteo (bone) and myo (muscular) anabolic activity. Binding and activation of the Androgen receptor alters the expression of genes and increases protein synthesis, hence builds muscle. So in essence, SARMs such as Osta Rx??? causes muscle growth in the same manner as steroids, however unlike testosterone and other anabolic steroids and prohormones, SARMs (as nonsteroidal agents) don???t produce the growth effect on prostate and other secondary sexual organs.
Osta Rx??? in particular exerts its anabolic effects on muscle tissue almost exclusively. So not only does it represent a new potential treatment option for a wide spectrum of conditions from muscle wasting diseases (from age-related to AIDS or cancer-related), but is also has immense potential for muscle building for Bodybuilders, fitness, athletes and an agent to minimize atrophy during recovery periods from serious surgery or similar situations.
Support Ingredients in Osta Rx???
Mucuna Pruriens ~ contains a very powerful neurotransmitter pre-cursor L-Dopa. Mucuna pruriens is a reputed remedy of Ayurveda in nervous and sexual diseases. Traditionally, Mucuna pruriens is commonly used as carminative, hypertensive and hypoglycemic agent. Mucuna pruriens has been found to contain L-DOPA, 40 mg/g of the plant. The plant/seeds contain the bioactive alkaloids mucunine, mucunadine, mucuadinine, pruriendine and nicotine, besides B-sitosterol, glutathione, lecithin, oils, venolic and gallic acids. Studies in experimental model show L-Dopa also helps in the reduction of cholesterol and blood sugar levels.
L-Dopa is an amino acid that converts into dopamine. Dopamine is an essential component of our body and it's required for proper functioning of the brain. Research discovered the body converts the amino acid tyrosine into L-dopa; L-dopa is then converted into dopamine. Without the neurotransmitter dopamine to serve a damping effect on neural transmissions, muscles become tense and tremble.
Benefits of Mucuna Pruriens L-Dopa:
-Improved sleep (promotes deep sleep)
-Reduced bodyfat & decreased cellulite
-Improved skin texture & appearance
-Increased bone density and reversal of osteoporosis
-Increased lean muscle mass
-Improved mood and sense of well-being
-Enhanced libido & sexual performance
-Increased energy levels
-Improved cholestorol profile & regeneration of organs (heart, kidney, liver, lungs)
-Dramatically strengthens immune system
Mucuna: Human Growth Hormone
L- Dopa contains natural secretagogues which may support the body's ability to stimulate the natural release of growth hormone. The blood carries the dopamine into the brain, where it naturally increases HGH production from the pituitary gland. The increased dopamine levels also optimize the production of other hormones, including testosterone, leading to increased sex drive and improved sexual performance for both men and women, beneficial in stimulating muscle growth, as well as burning fat from fat cells.
Fenuside ~ is a testosterone booster containing Fenuside saponins, extracted from the herb Fenugreek (Trigonella foenum-graecum). Fenuside is designed to boost testosterone levels, muscle size and sex drive, and is considered one of the very latest testosterone boosters in the sports supplement market. It is important to note that there are over 100 natural chemicals in Fenugreek, but it's only standardised Fenuside saponins that are proven to offer bodybuilders, gym users and athletes beneficial effects on muscle size, testosterone levels and body composition.
The fenuside saponins found in Fenuside are designed to support hormone levels and act as a powerful but safe testosterone booster in individuals desiring fast and noticeable enhancements in muscle size, strength and performance. The supplement is useful for strength and power athletes, body builders, and serious gym users.
Research suggests that Fenuside mechanism of action is initially as an adrenal cortex stimulator, subsequently activating the hypothalamus and boosting natural production of corticotropin releasing hormone (CRH). CRH switches on the powerful pituitary gland, enhancing production of the key adrenocorticotrophin hormone (ACTH). ACTH is a potent stimulant on the adrenal cortex to increase androgen synthesis. Because androgens are precursors to Testosterone and possess "Testosterone like activity", Testofen naturally supports the activity of the luteinizing hormone, acting as a testosterone booster.
Horny Goat Weed ~ Icariin is the active element of Epimedium Extract (also commonly known as Horny Goat Weed Extract) and this ingredient when extracted to high purity's is an exceptionally powerful nitric oxide and testosterone booster. Icariin is a very fine grade of extract and boasts quality's which simply can't be obtained from the lower grade's of Epimedium Extract. The increased blood flow and oxygen to the muscles obtained from Icariin of this quality feeds the body with the energy and the drive required to perform and out-perform when under activities of physical and mental endurance.
Selective Androgen Receptor Modulator (SARM)
-Non-hormonal Anabolic Compound
-Increases Lean Muscle Mass
-Promotes Fat Loss
-Promotes Recovery
-Increases Libido
-Safe for Males & Females
-Can be used for PCT and Bridging
IronMagLabs Bodybuilding Supplements & Prohormones: Osta Rx
(MK-2866) ~ ((2S)-3-(4-cyanophenoxy)-N-[4-cyano-3-(trifluoromethyl)phenyl]-2-hydroxy-2-methylpropanamide)

Osta Rx??? is a Selective Androgen Receptor Modulator. A SARM is exactly what it sounds like: a compound (not an anabolic steroid) which has the ability to stimulate the androgen receptor (much the same way as anabolic steroids). Osta Rx??? is an orally active (and highly bioavailable) selective agonist for androgen receptors which was shown to have anabolic effects in muscle and bone tissue. It has been shown to have no measurable effect on lutenizing hormone (LH) or follicle-stimulating hormone (FSH), but it has been shown to have some effect on prostate weight, with an androgenic potency around 1/3rd of its anabolic potency. Still, this is a good trade-off, because it???s anabolic effect has been measured to be roughly the same as testosterone. It has also been shown to produce dose-dependent increases in bone mineral density and mechanical strength in addition to being able decrease body fat and
increase lean body mass.

Selective androgen receptor modulators (SARMs) bind to the androgen receptor and demonstrate osteo (bone) and myo (muscular) anabolic activity. Binding and activation of the Androgen receptor alters the expression of genes and increases protein synthesis, hence builds muscle. So in essence, SARMs such as Osta Rx??? causes muscle growth in the same manner as steroids, however unlike testosterone and other anabolic steroids and prohormones, SARMs (as nonsteroidal agents) don???t produce the growth effect on prostate and other secondary sexual organs.
Osta Rx??? in particular exerts its anabolic effects on muscle tissue almost exclusively. So not only does it represent a new potential treatment option for a wide spectrum of conditions from muscle wasting diseases (from age-related to AIDS or cancer-related), but is also has immense potential for muscle building for Bodybuilders, fitness, athletes and an agent to minimize atrophy during recovery periods from serious surgery or similar situations.
Support Ingredients in Osta Rx???
Mucuna Pruriens ~ contains a very powerful neurotransmitter pre-cursor L-Dopa. Mucuna pruriens is a reputed remedy of Ayurveda in nervous and sexual diseases. Traditionally, Mucuna pruriens is commonly used as carminative, hypertensive and hypoglycemic agent. Mucuna pruriens has been found to contain L-DOPA, 40 mg/g of the plant. The plant/seeds contain the bioactive alkaloids mucunine, mucunadine, mucuadinine, pruriendine and nicotine, besides B-sitosterol, glutathione, lecithin, oils, venolic and gallic acids. Studies in experimental model show L-Dopa also helps in the reduction of cholesterol and blood sugar levels.
L-Dopa is an amino acid that converts into dopamine. Dopamine is an essential component of our body and it's required for proper functioning of the brain. Research discovered the body converts the amino acid tyrosine into L-dopa; L-dopa is then converted into dopamine. Without the neurotransmitter dopamine to serve a damping effect on neural transmissions, muscles become tense and tremble.
Benefits of Mucuna Pruriens L-Dopa:
-Improved sleep (promotes deep sleep)
-Reduced bodyfat & decreased cellulite
-Improved skin texture & appearance
-Increased bone density and reversal of osteoporosis
-Increased lean muscle mass
-Improved mood and sense of well-being
-Enhanced libido & sexual performance
-Increased energy levels
-Improved cholestorol profile & regeneration of organs (heart, kidney, liver, lungs)
-Dramatically strengthens immune system
Mucuna: Human Growth Hormone
L- Dopa contains natural secretagogues which may support the body's ability to stimulate the natural release of growth hormone. The blood carries the dopamine into the brain, where it naturally increases HGH production from the pituitary gland. The increased dopamine levels also optimize the production of other hormones, including testosterone, leading to increased sex drive and improved sexual performance for both men and women, beneficial in stimulating muscle growth, as well as burning fat from fat cells.
Fenuside ~ is a testosterone booster containing Fenuside saponins, extracted from the herb Fenugreek (Trigonella foenum-graecum). Fenuside is designed to boost testosterone levels, muscle size and sex drive, and is considered one of the very latest testosterone boosters in the sports supplement market. It is important to note that there are over 100 natural chemicals in Fenugreek, but it's only standardised Fenuside saponins that are proven to offer bodybuilders, gym users and athletes beneficial effects on muscle size, testosterone levels and body composition.
The fenuside saponins found in Fenuside are designed to support hormone levels and act as a powerful but safe testosterone booster in individuals desiring fast and noticeable enhancements in muscle size, strength and performance. The supplement is useful for strength and power athletes, body builders, and serious gym users.
Research suggests that Fenuside mechanism of action is initially as an adrenal cortex stimulator, subsequently activating the hypothalamus and boosting natural production of corticotropin releasing hormone (CRH). CRH switches on the powerful pituitary gland, enhancing production of the key adrenocorticotrophin hormone (ACTH). ACTH is a potent stimulant on the adrenal cortex to increase androgen synthesis. Because androgens are precursors to Testosterone and possess "Testosterone like activity", Testofen naturally supports the activity of the luteinizing hormone, acting as a testosterone booster.
Horny Goat Weed ~ Icariin is the active element of Epimedium Extract (also commonly known as Horny Goat Weed Extract) and this ingredient when extracted to high purity's is an exceptionally powerful nitric oxide and testosterone booster. Icariin is a very fine grade of extract and boasts quality's which simply can't be obtained from the lower grade's of Epimedium Extract. The increased blood flow and oxygen to the muscles obtained from Icariin of this quality feeds the body with the energy and the drive required to perform and out-perform when under activities of physical and mental endurance.
Attachments
Last edited by a moderator:







